Weed Research (1999) 39:107-115
SEASONAL CHANGES IN THE GERMINATION OF
BURIED SEEDS OF MONOCHORIA VAGINALIS
PH Chen & WHJ Kuo
Department of Agronomy, National Taiwan University
Taipei 10764, Taiwan
Germination of buried
Monochoria seeds
Corresponding author: W.H.J. Kuo
Department of Agronomy
National Taiwan University
Taipei 10764, Taiwan
Tel: 886-2-3630231 ext. 2728
Fax: 886-2-3620879
Email: whjkuo@ntu.edu.tw
Summary
This study investigates the seasonal variation of germination ability of buried seeds
of Monochoria vaginalis (Burm.f.) Presl var. plantaginea Solms. The
field-collected seeds were buried in a flooded or an upland field and then exhumed
monthly. The exhumed seeds were germinated under four temperature regimes. The seeds
exhumed from the flooded soil were dormant at the beginning of burial, and proceeded into
conditional dormancy–non-dormancy–conditional dormancy cycle throughout the remaining
period of the experiment. The seeds exhumed monthly from the non-flooded soil exhibited an
annual dormant cycle, which is dormancy–conditional dormancy–non-dormancy–conditional
dormancy–dormancy. At day and night temperatures of 25/20 oC, the exhumed
seeds from both the flooded and upland soil resembled each other in terms of seasonal
variation of the germination percentage. In September and October, more seeds exhumed from
upland soil failed to germinate under higher temperature than from flooded soil. Strictly
avoiding exposure to light during seed exhuming and seed testing prevented the seeds from
germinating. A short exposure of the exhumed seeds to light during preparation promoted
dark germination when the seeds were at the non-dormant stage. The potential implications
of our results for weed management strategies in rice production are discussed.
Keywords: buried seed, dormancy, germination, Monochoria
vaginalis, rice
Introduction
Monochoria vaginalis has been listed as a serious weed in six countries, including Borneo, Indonesia, Japan, Korea and Taiwan
(Holm et al., 1979). A recent investigation rated M. vaginalis as the worst
weed in Southeast Asia next to Echinochloa colona (Waterhouse, 1993). In Taiwan, it
is one of the five most serious weeds in paddy fields (Chiang & Leu, 1982). Although
easily killed by butachlor during seedling stage (Liu & Tsai, 1986), M. vaginalis
remains a serious weed. In light of this predicament, further knowledge of seed
germination biology is required if effective management strategies are to be proposed.
Elucidating the dormancy cycle of M. vaginalis is a prerequisite for
understanding the year round behaviour of the seeds in the soil. According to Baskin &
Baskin (1985), dormant seeds are defined as seeds not germinating at any temperature
regime. In the soil, these seeds gradually become conditionally dormant, capable of
germinating at relatively narrow temperature regimes. The conditionally dormant seeds may
enter into a non-dormant state as long as they remain quiescent in the soil. The
non-dormant seeds germinate at a wider range of temperatures. Other soil factors, e.g.
allelopathic inhibitors, lack of light or oxygen, or extreme temperatures may inhibit the
germination of the non-dormant or conditionally dormant seeds in the soil. Non-germinated
seeds may proceed into conditional dormancy and then back into the dormant state again.
The germination characteristics of seeds of temperate species have received extensive
interest (Baskin & Baskin, 1988, 1989a; Probert, 1992). Although seed ecology of paddy
weeds has gained lesser attention, the ecology of wetland seed banks has been thoroughly
reviewed (Leck, 1989). This study surveys the changes in dormant states of buried seeds of
M. vaginalis.
Materials and Methods
Seeds
The seeds of M. vaginalis (Burm.f.) Persil var. plantaginea Solms. were
collected on the experimental farm of the National Taiwan University in Taipei in October
1993 and May 1994. Empty and under-developed seeds were discarded by floating in tap
water. The remaining seeds were air-dried to about 8.5% moisture content (wet basis) and
hermetically stored in a -20 oC chest cabinet to keep the seeds fresh. At the
time of collection, the seeds were dormant and storage at -20 oC was therefore
assumed not to influence the dormancy status of the seeds.
Burial experiment I (BE I)
In November 1993, after one month of storage at -20 oC, the seeds collected
in October 1993 were divided into 48 samples and packed separately in 4 cm x 6 cm bags of
fine mesh nylon gauze (approximately 2000 seeds per bag). Half of the bags were buried
horizontally in a loam soil under non-irrigated upland condition. The other 24 bags were
buried in a loam soil under water-logged condition. The water level was maintained 5-10 cm
above soil surface during the experiment. The depth of burial was approximately 10 cm.
Burial experiment II (BE II)
In June 1994, the seeds collected in May 1994 were divided into 24 samples and packed
and buried as in BE I, except that the envelopes were buried in sandy loam in a black
plastic pot that were lined with gauze to prevent loss of soil from the pot. The pots were
then buried in upland or paddy field at a depth so that the envelopes were approximately
10cm below soil surface. This procedure prevented light from reaching the seeds during
exhuming and when preparing the seeds for the germination test.
Germination tests
One bag of seeds was exhumed monthly from each experiment. No
light-preventing procedure was adopted in BE I during transportation and processing of the
seeds, and when counting the numbers of germinated seeds. In contrast, in BE II, the
exhumed pots were covered with a black polyethylene bag and then moved into a dark room
that was equipped with a dim green light (< 0.01 micro mol/m2/s). All the
seed handling procedures were performed in the dark room.
The exhumed seeds were water-washed and air-dried for one day in the
laboratory (BE I) or in a dark room (BE II). Thirty-two petri dishes each with 50 seeds
were prepared for each bag. The seeds were placed on two sheets of filter paper and 10 ml
distilled water were added. Petri dishes were wrapped with parafilm to prevent loss of
water. Half of the petri dishes were subjected to a 16 hours dark period and 8 hours light
period daily. Light was provided by fluorescent tubes at an intensity of about 30 micro
mol/m2/s at water surface (4 mm above seed surface). To create a completely
dark condition the other half of the petri dishes were sealed by two layers of aluminum
bag. This test method maintained the seeds under partially anaerobic conditions, which
promoted germination (Momonoki, 1992; Chen & Kuo, 1995). Germination tests were
performed in incubators at the following day/night temperature regimes: 30/25, 30/20,
25/20 and 23/13oC.
A seed was considered germinated if the embryo axes protruded more than
2 mm. The time of the first count depended on germination temperature; the final count was
made at the 14th day of incubation. Mean germination time at 16 oC has
previously been found to be 7 days for non-dormant seeds (Chen & Kuo, 1995).
Field emergence
Monthly exhumed seeds from both BE I and BE II were sown on a
water-logged field 0.5 cm below the soil surface. The topsoil had been autoclaved to kill
all seeds. Burial at 0.5 cm did not significantly reduce germination compared to un-buried
Monochoria seeds (Chen & Kuo, 1995). Seedling counting was terminated one month
after sowing.
Meteorological data
Daily mean soil temperature at 10cm below soil surface was calculated
from the data recorded at 30 min. interval by a data logger.
Results
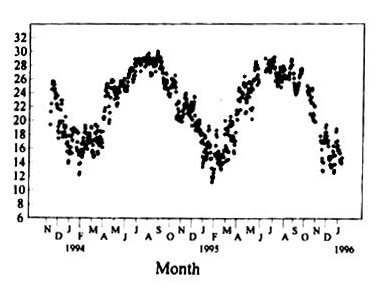
The daily mean soil temperature in the paddy fields (10 cm below
surface) in Taipei during the experiments is shown in Figure 1. Mean soil temperature
exhibited a typical annual fluctuation, highest mean temperature ranging from 28-30 oC
occurred during June and September, while in the winter, it was as low as 12-14 oC.
Mean upland soil temperature at 10 cm below surface did not significantly differ from that
of the paddy field although the amplitudes of daily alternating temperature always
exceeded that of the paddy field (data not shown). During autumn and early winter
precipitation was less than during spring and summer. Nevertheless, the soils always
remained wet during autumn and winter due to lower radiation and temperatures. In
contrast, during the summer, the topsoil dried out on a few sunny days.
Figure
1, Daily mean temperature at 10 cm below a flooded soil surface at Taipei during the
experiments.
|
 |
The results of BE I showed that all M. vaginalis seeds collected
in October 1993 and stored at -20oC for one month were dormant at all four
temperature regimes. Although still very high after one month of burial in water-logged
soil (Fig. 2a), the dormancy level was greatly reduced after two months of burial.
Thereafter, 60–90% of the seeds germinated at 30/25 oC except those exhumed
in October 1995. At 30/20 oC, the germination percentage was 30-80%. Comparing
these two temperature regimes revealed a greater difference in germination during winter
than during summer. At 25/20 oC, the germination pattern revealed a seasonal
fluctuation. Germination increased from winter until early summer, and then decreased
again until autumn. At 23/13 oC germination never exceeded 20% throughout the
experiment.
Figure
2, Percentage germination under light (A) and dark (B) condition of Monochoria
vaginalis seeds exhumed monthly from a flooded soil (BE I). Vertical bars indicate
standard error, if greater than 5%. Germination tests were performed at 30/25 (n ), 30/20 (l ), 25/20 (u ), and 23/13 oC (△). No exhumation was made in May, June, August and December 1995.
During exhuming, the seeds were not strictly prevented from exposure to light.
|
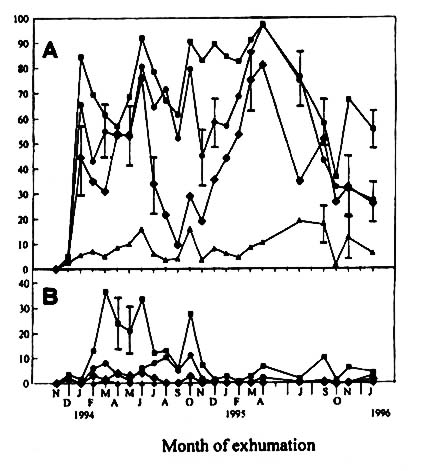 |
From March to June of the first year of paddy-field burial, 20 - 40% of
the seeds germinated at 30/25 oC under the dark condition (Fig. 2b). The lower
germination percentage under dark condition indicated that these seeds were very sensitive
to light exposure during exhuming. One year after the burial a short exposure to light did
not stimulate germination.
At 25/20oC the annual fluctuation in percentage germination
of the exhumed seeds buried in upland soil (Fig. 3a) resembled those buried in the paddy
fields. However, in the upland soil, the seeds became completely dormant during summer, as
revealed by low germination percentage at 30/25oC. The seeds gradually
proceeded into conditional dormancy during autumn and winter and became non-dormant in
spring of the next year. According to dark germination tests (Fig. 3b) up to 30% of the
seeds lost their light requirement in the first spring.
Figure
3, Percentage germination of Monochoria vaginalis seeds exhumed monthly from
upland soil (BE I). Legends as in Figure 2.
|
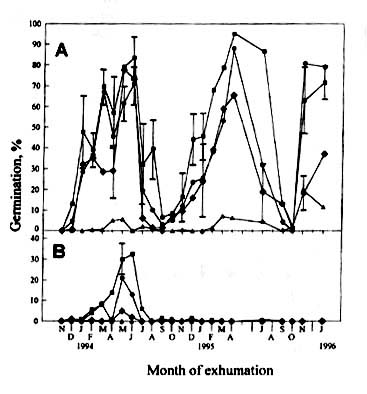 |
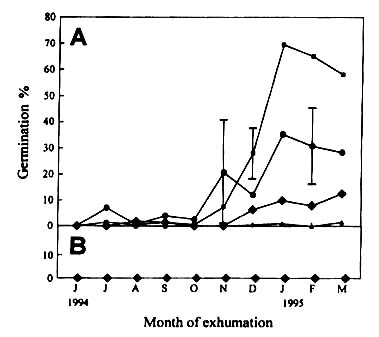
Although the BE II experiments did not last long enough to reveal any
dormancy cycle, our results nevertheless indicated that the seeds buried in summer
maintained a longer dormancy period (Figs. 4 and 5) than those buried in winter (Figs. 2
and 3). Again, the seeds acquired the ability to germinate quicker when buried in the
paddy field than in upland soil. In contrast to BE I, in BE II no seeds germinated under
dark condition (Figs. 4b and 5b).
Figure
4, Percentage germination of Monochoria vaginalis seeds exhumed monthly from a
flooded soil (BE II). The seeds were protected from light during exhumation and the seeds
for the dark germination test were prepared under green safe light. Legends as in Figure
2.
|
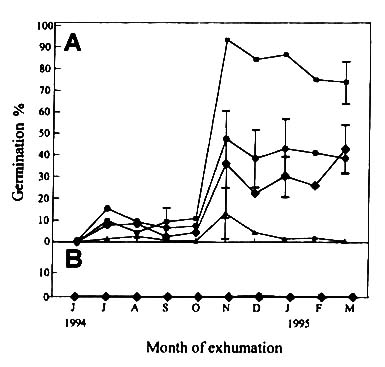 |
Figure
5, Percentage germination of Monochoria vaginalis seeds exhumed monthly from
upland soil (BE II). The seeds were kept from light during exhumation and the seeds for
the dark germination test were prepared under green safe light. Legends as in Figure 2.
|
 |
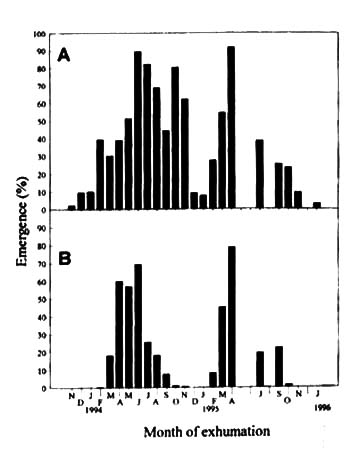
The seeds that were exhumed from water-logged soil did not germinate in
the field at the beginning (Fig. 6a). In the two years survey, percentage emergence of the
exhumed seeds gradually increased up to 90% during spring. The percentage then decreased
during summer, and was lowest in winters, with the exception of October and November 1994.
Seeds exhumed from upland soil also displayed an annual change in percentage field
emergence (Fig. 6b), but were generally more dormant except from April to June 1994 and in
April 1995.
Figure
6, Percentage emergence of Monochoria vaginalis seeds exhumed monthly from a
flooded (A) and an upland (B) soil and then re-planted into a water-logged field. No
exhumation was made in May, June, August and December 1995.
|
 |
Discussion
The seeds of M. vaginalis are easily separated from the fruits,
the average length is less than 2 mm, and the mean seed weight is around 0.17 mg.
According to Grime (1989), these characteristics imply that the seeds constitute a
persistent seed bank in the soil. Our one year survey did indicate that M. vaginalis
seeds always appear in the same paddy field (unpublished results). Similar to other tiny
persistent seeds, the non-dormant seeds of M. vaginalis failed to germinate in the
dark. However, the seeds are highly sensitive to dim light. Two days of one-minute
exposure to light at an intensity of 4 x 10 –4 mol/m2
were sufficient to promote 50 % of the dormant M. vaginalis seeds to germinate
(Chen & Kuo, 1995). This low level of light intensity also promoted the emergence of
some dicotyledonous weed seeds, which were subjected to a single tillage in night under
artificial light and then buried again (Scopel et al., 1994).
Some seeds exhibit a fluctuation in light requirement during burial,
e.g. Capsella bursa-pastoris Medik.(Baskin & Baskin, 1989b), Polygonum
aviculare L. (Baskin & Baskin, 1990), Oenothera biennia L. (Baskin
& Baskin, 1994), and Carex stricta Lam.(Baskin et al., 1996) On the
other hand, some species maintain light requirement during the entire period of burial,
such as the seeds of the wetland plants Cyperus erythrorhizos Muhl., Cyperus
flavicomus Michx., Fimbristylis autumnalis (L.) Roem. &
Schult. (Baskin et al., 1993), as well as Carex comosa F. Bott seeds buried at non-flooded condition
(Baskin et al., 1996). Exhumed seeds of these species do not germinate in darkness.
The M. vaginalis seeds did not lose their light requirement for
germination during burial (Figs. 4b and 5b). However, up to 30% of the buried seeds
responded to a short period of light exposure during exhuming in the spring and early
summer of 1994 (Figs. 2b and 3b). Thereafter the seeds required more light for
germination. Flooding somewhat decreased the light requirement of M. vaginalis
seeds. In contrast, the effect of flooding on overcoming seed dormancy of C. comosa
was much more significant (Baskin et al., 1996).
Our results clearly indicated that the buried seeds of M. vaginalis
in paddy fields experienced annual changes in dormant state (Fig. 2a). The seeds were
dormant at the beginning of the burial, became conditionally dormant in January, went into
non-dormant state in April, became conditionally dormant again in July and remained so
until March 1995. This annual dormancy cycle was not perfectly repeated during 1995. Only
40% of the seeds germinated at 30/25 oC around August 1995, while in 1994 the
germination percentage was 60-90%. Germination percentage at the lowest temperature regime
(25/20 oC) fluctuated throughout the experiment in contrast to 30/25 oC,
when germination percentage remained high for most of the burial period. This fluctuation
pattern reflects the characteristic of a strictly summer annual species (Karssen, 1982;
Baskin & Baskin, 1989a). Notably the conditional dormancy–non-dormancy–conditional
dormancy cycles of M. vaginalis resembled that of the seeds of the wetland
perennials Gratiola viscidula Pennell and Scirpus lineatus Michx. (Baskin et
al., 1989) as well as that of facultative winter annual or spring- and summer-
germinating summer annuals (Baskin & Baskin, 1989a).
Seeds performed differently when buried in the upland soil (Fig. 3a),
exhibiting a dormancy– conditional dormancy– non-dormancy– conditional dormancy–
dormancy cycle, resembling that of a spring germinating summer annual, P. aviculare
(Baskin & Baskin, 1990). Interestingly, most of the M. vaginalis seeds that
were exhumed in September and October from upland soil failed to germinate at 30/25 oC,
while those from the paddy field germinated under the same conditions. It suggests that
the drying condition of the soil during summer may induce complete dormancy of the buried
seeds.
Although the seeds of Carex species were less dormant in flooded
than in non-flooded soil, the basic dormant cycle did not differ much (Baskin et al.,
1996). According to their results, the seeds of some other Cyperaceae species
entered conditional dormancy in non-flooded condition, but not when buried in flooded soil
(Baskin et al., 1993). The above findings suggest that drying may induce dormancy
in buried seeds of aquatic species, which may be of ecologically significance. Dormancy of
the non-dormant M. vaginalis seeds can be partially induced by drying in the
laboratory. However, up to 80% of the artificially dried seeds germinated when tested at
30/25 oC (data not shown). The cause of the drastic decrease in percentage
germination at 30/25 oC of the non-flooded seeds that were exhumed in September
or October remains unexplained.
In Taiwan there are two rice crops per year. In the northern part of
the island, the first crop is grown from March to July and the second crop from August to
November. In practice, the seed behaviour of M. vaginalis more resembles that of a
spring and summer germinating summer annual weed, because M. vaginalis emerges from
the field in late spring when temperature regimes are around 30/25 oC. The weed
plants are killed at the time of rice harvesting and subsequent land preparation. During
the summer rice season, the seeds germinate at the beginning of transplanting. The weed
plants again are killed at harvest. On the other hand, field observations have shown that
the plant can survive the winter climate, behaving like perennial or at least biennial as
long as the paddy field remains water-logged all year round (Zimdahl et al., 1989).
Consequently M. vaginalis closely resembles G. viscidula and S. lineatus
and incidentally, the seeds of the last two species are also small and light dependent
(Baskin et al., 1989).
A large portion of the M. vaginalis seeds in the soil losses
their dormancy after March. These seeds would germinate promptly after land preparation
provided the soil is well flooded and the seeds had been shortly exposed to light. These
characteristics render the weed easy to control, but hard to eliminate. However, our data
indicate that several times of tillage in late spring would minimise the weed population
efficiently. The germination pattern also reveals a promising means of controlling this
weed in the second rice crop; in order to induce completely dormancy of the M.
vaginalis seeds, the soil should be allowed to be completely dried out for a short
period between the two rice crops. Incorporating land preparation and desiccation
procedure in the cropping practice could contribute to a more effective weed control
strategy.
Acknowledgements
The second author would like to thank the National Science Council of
Taiwan for financially supporting this research under Contract No. NSC NSC83-0409-B002-26,
as well as Dr. Birgit Voigt of the Botanisches Institut, E.-M.-Arndt-Universitat, for her
comments.
References
Baskin JM & Baskin CC (1985) The annual
dormancy cycle in buried weed seed: A continuum. Bioscience 35, 492-498.
Baskin CC & Baskin JM (1988) Germination
ecophysiology of herbaceous plant species in a temperate region. American Journal of
Botany 75, 286-305.
Baskin CC & Baskin JM (1989a) Physiology of dormancy
and germination in relation to seed bank ecology. In: Ecology of Soil Seed Bank
(eds. M.A. Leck, V.T. Paker & R.L. Simpson), pp. 53-66. Acad. Press, San Diego.
Baskin JM & Baskin CC (1989b) Germination responses of buried seeds
of Capsella bursa-pastoris exposed to seasonal temperature changes. Weed
Research 29, 205-212.
Baskin JM & Baskin CC (1990) The role of light and
alternating temperature on germination of Polygonum aviculare seeds exhumed on
various dates. Weed Research 30, 397-402.
Baskin CC & Baskin JM (1994) Germination
requirements of Oenothera biennia seeds during burial under natural seasonal
temperature cycles. Canadian Journal of Botany 72, 779-782.
Baskin JM, Baskin CC & Spooner DM (1989) Role of
temperature, light and date: seeds were exhumed from soil on germination of four wetland
perennials. Aquatic Botany 35, 387-394.
Baskin JM, Baskin CC & Chester EW (1993) Seed
germination ecophysiology of four summer annual mudflat species of Cyperaceae. Aquatic
Botany 45, 41-52.
Baskin CC, Chester EW & Baskin JM (1996) Effect of
flooding on annual dormancy cycles in buried seeds of two wetland Carex species. Wetlands
16, 84-88.
Chen PH & Kuo WHJ (1995) Germination conditions for
the non-dormant seeds of Monochoria vaginalis. Taiwania 40, 419-432.
Chiang MY & Leu LS (1982) Weed and weed damage of
paddy field in Taiwan. Weed Science Bulletin (Taiwan) 3, 18-46.
Grime JP 1989 Seed banks in ecological perspective. In: Ecology
of Soil Seed Banks (eds. M.A. Leck, V.T. Parker & R.L. Simpson), pp. xv-xxii.
Academic Press, San Diego.
Holm L, Pancho JV, Herberger JP & Plucknett DL
(1979) A Geographical Atlas of World Weed. John Wiley & Son, New York.
Karssen CM 1982 Seasonal patterns of dormancy in weed
seeds. In: The Physiology and Biochemistry of Seed Development, Dormancy and
Germination (ed. A.A. Khan), pp. 243-270. Elsevier Biomedical Press, Amsterdam.
Leck MA (1989) Wetland seed banks. In: Ecology of
Soil Seed Banks (eds. M.A. Leck, V.T. Parker & R.L. Simpson), pp. 283-305.
Academic Press, San Diego.
Liu A & Tsai WF (1986) Effect of butachlor on the
germination and seedling growth of rice and some paddy weeds. Journal of the
Agriculture Association of China New series, (135) 1-9.
Momonoki YS 1992 Effect of ethylene and carbon dioxide on seed
germination of Monochoria vaginalis var. plantaginea. Weed Research (Japan)
37, 121-128.
Probert RJ 1992 The role of temperature in germination ecophysiology.
In: Seeds: The Ecology of Regeneration in Plant Communities (ed. M. Fenner),
pp.285-325. CAB International, Wallingford.
Scopel AL, Ballare CL & Radosecich SR (1994)
Photostimulation of seed germination during soil tillage. New Phytologist 126,
145-152.
Waterhouse DF (1993) Prospects for Biological Control
of Paddy Weeds in Southeast Asia and Some Recent Successes in the Biological Control of
Aquatic Weeds. Food & Fertilizer Technology Center, Extension Bulletin, No. 366,
Taipei.
Zimdahl RL, Lubigan RT, Moody K & Mabbayad MO (1989) Seeds and
Seedlings of Weeds in Rice in South and Southeast Asia. IRRI, Manila.





